Our MitoXcel™ geropeptide lead clinical candidates PTC-2105 and PTC-2107, are:
-
18- to 30-amino acids in length
-
Cross the blood brain barrier
-
Administered via SC injection over an 8-16 week and longer course of treatment in pre-clinical models
Key findings:
-
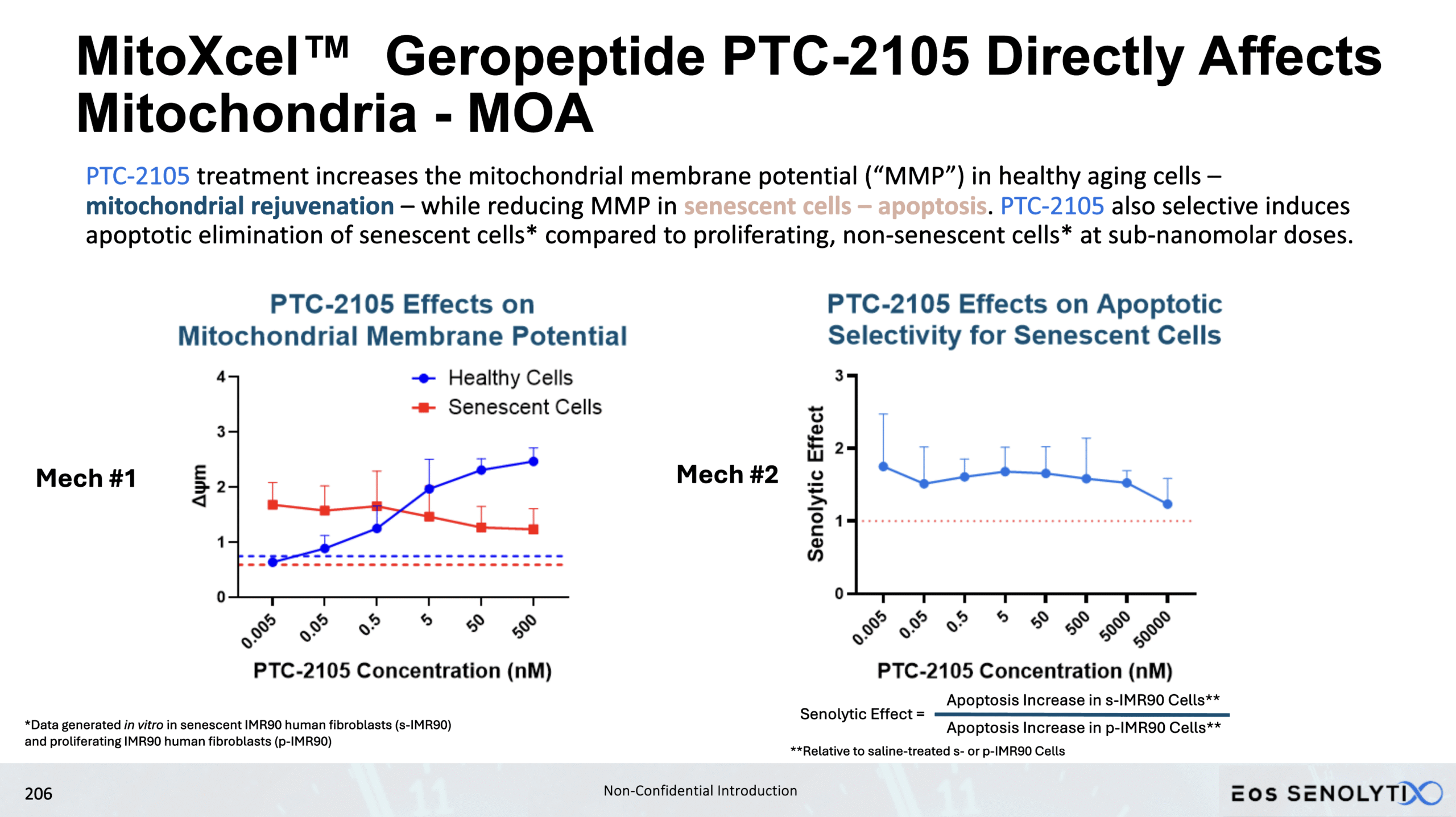
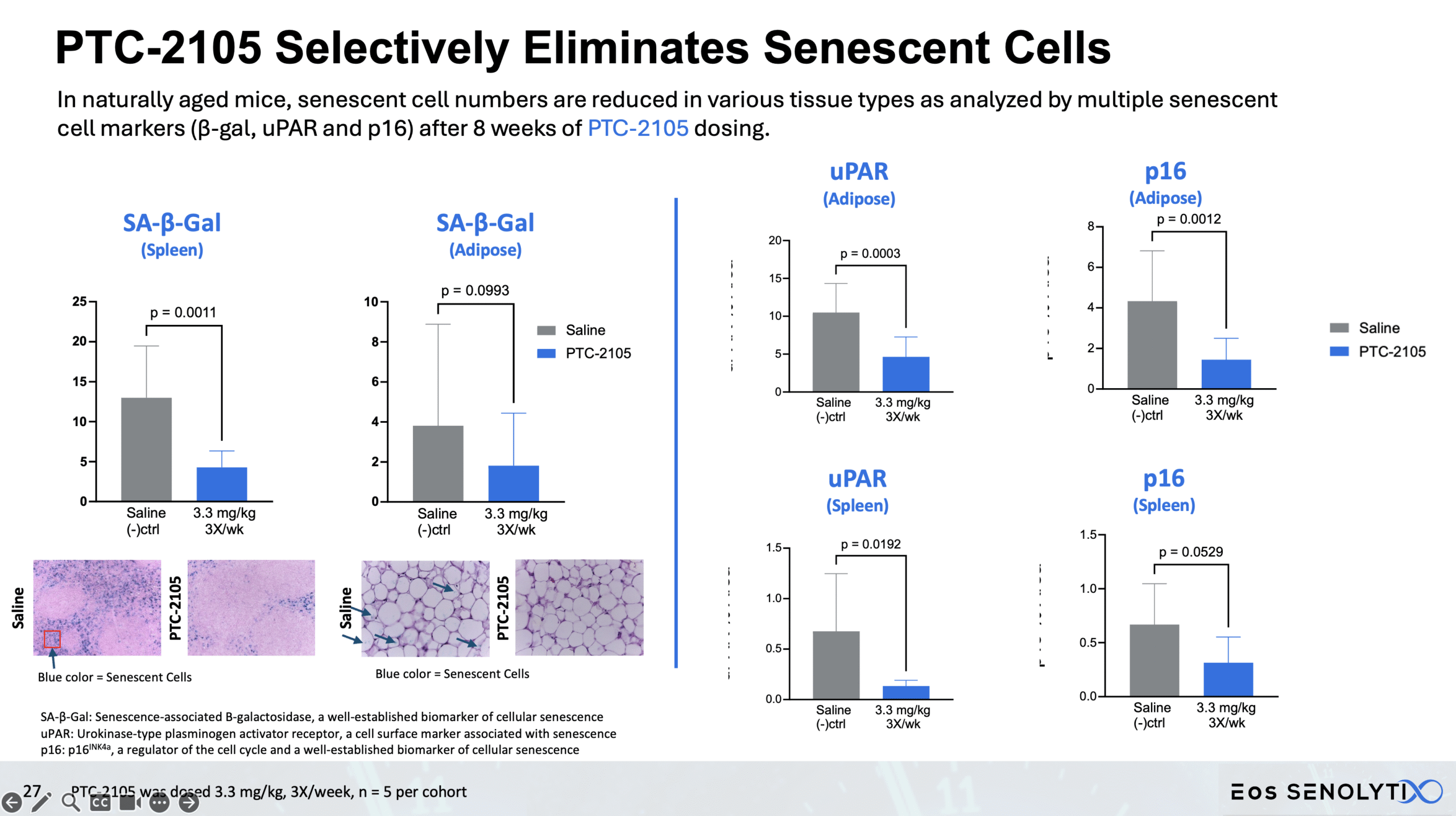
Selective Elimination of Senescent Cells: In all organs of the body, including the brain, in a dose dependent manner
-
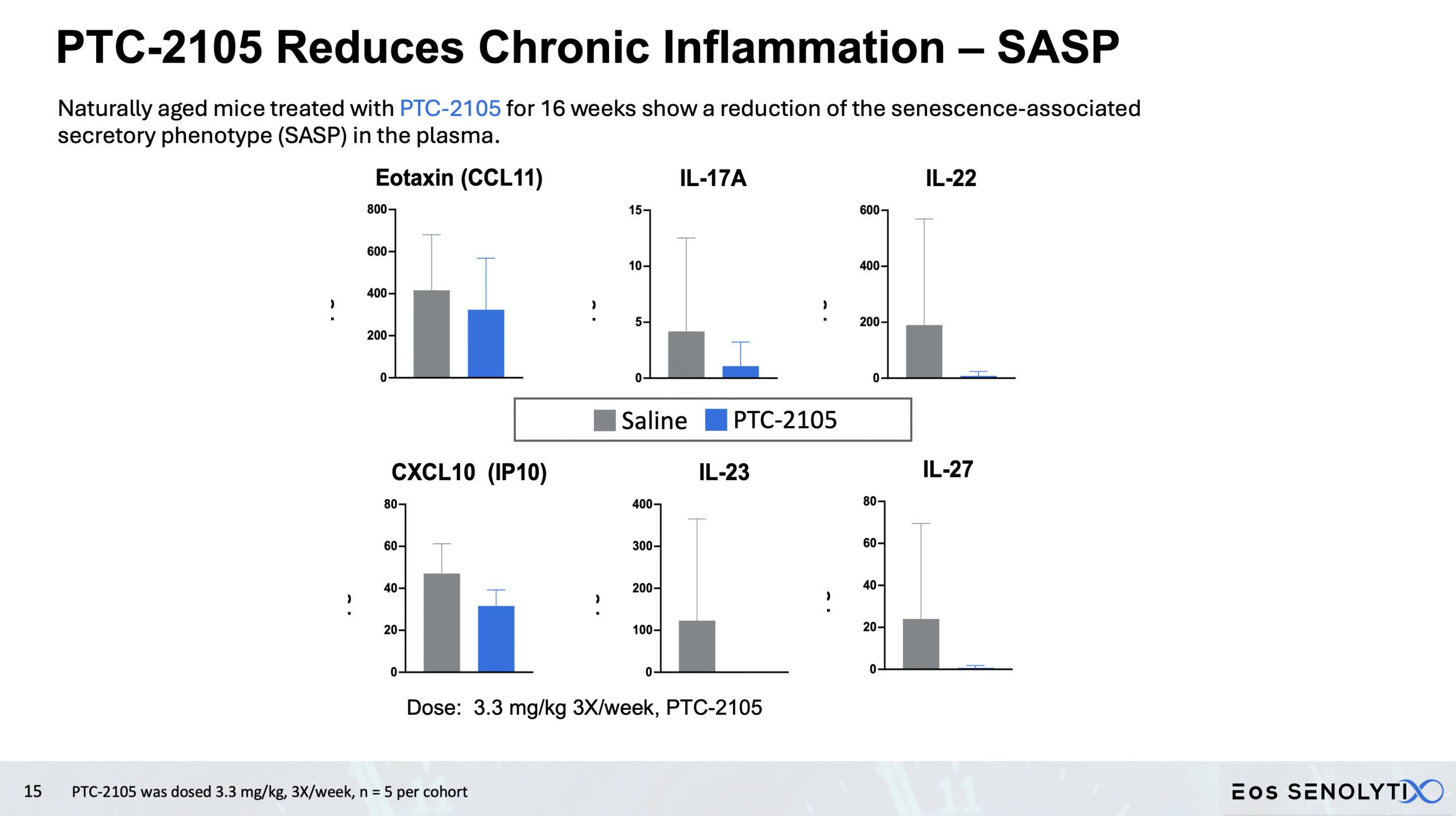
Reduced Systemic Inflammation: Significant decrease in SASP biomarkers
-
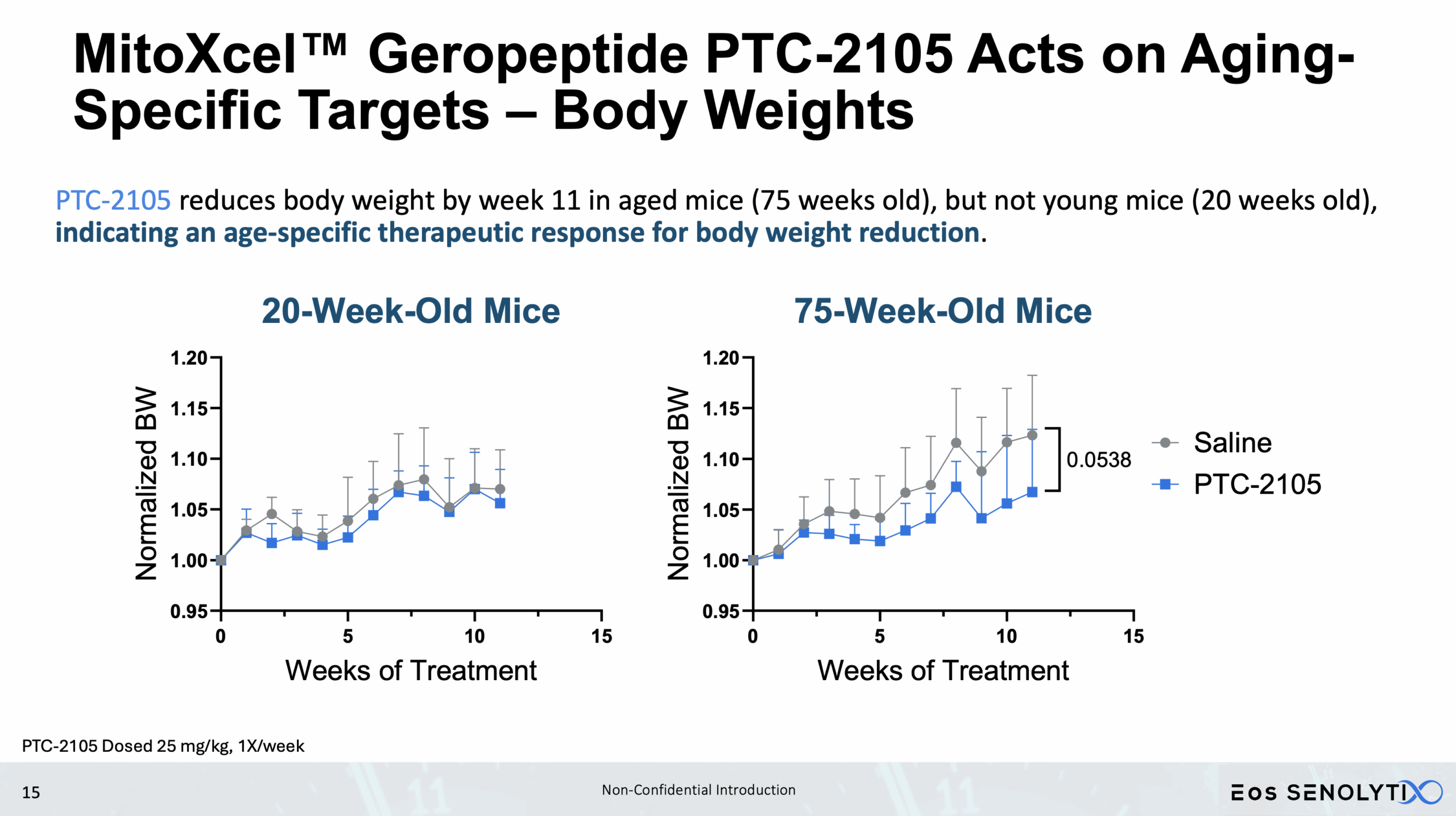
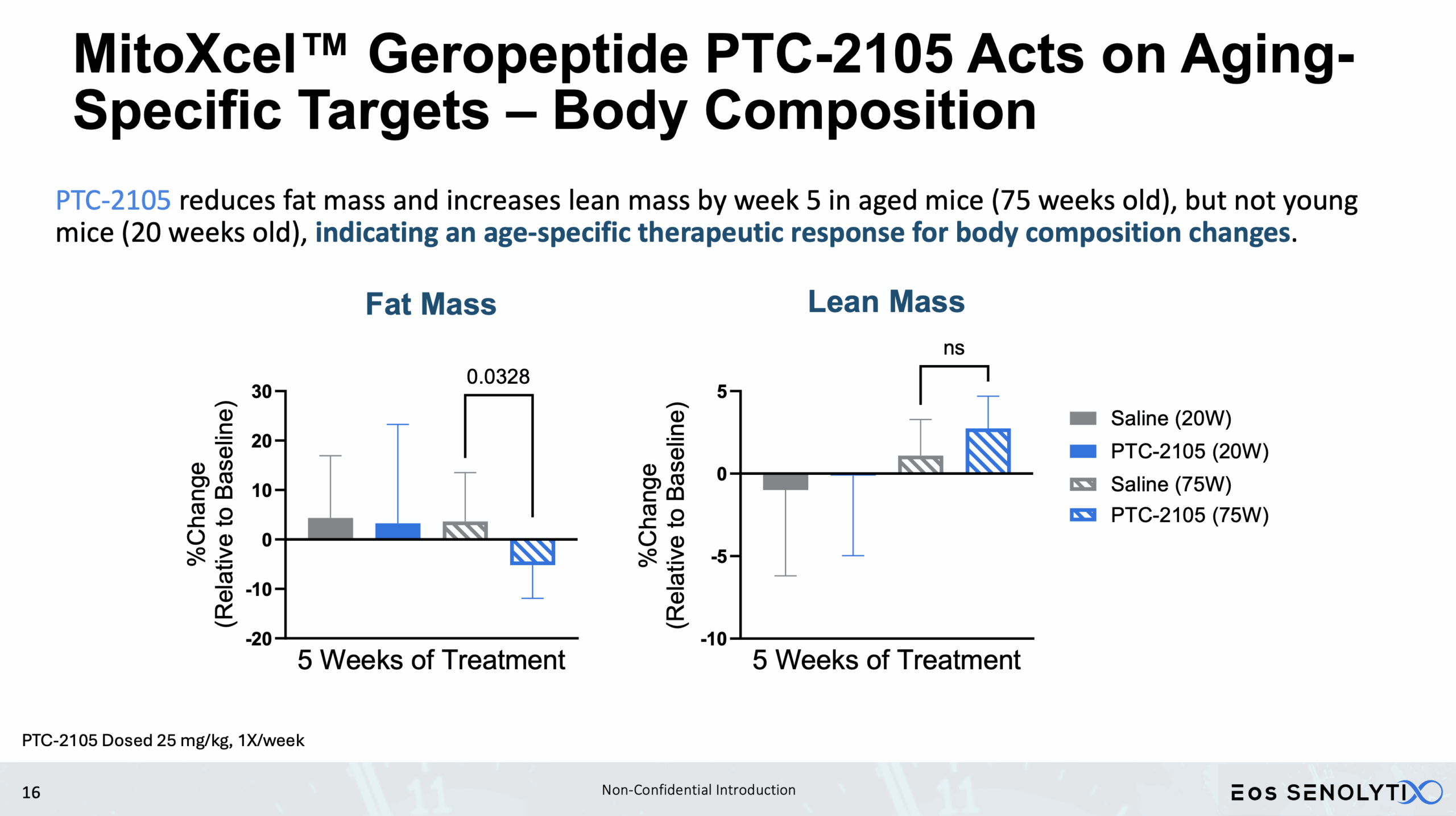
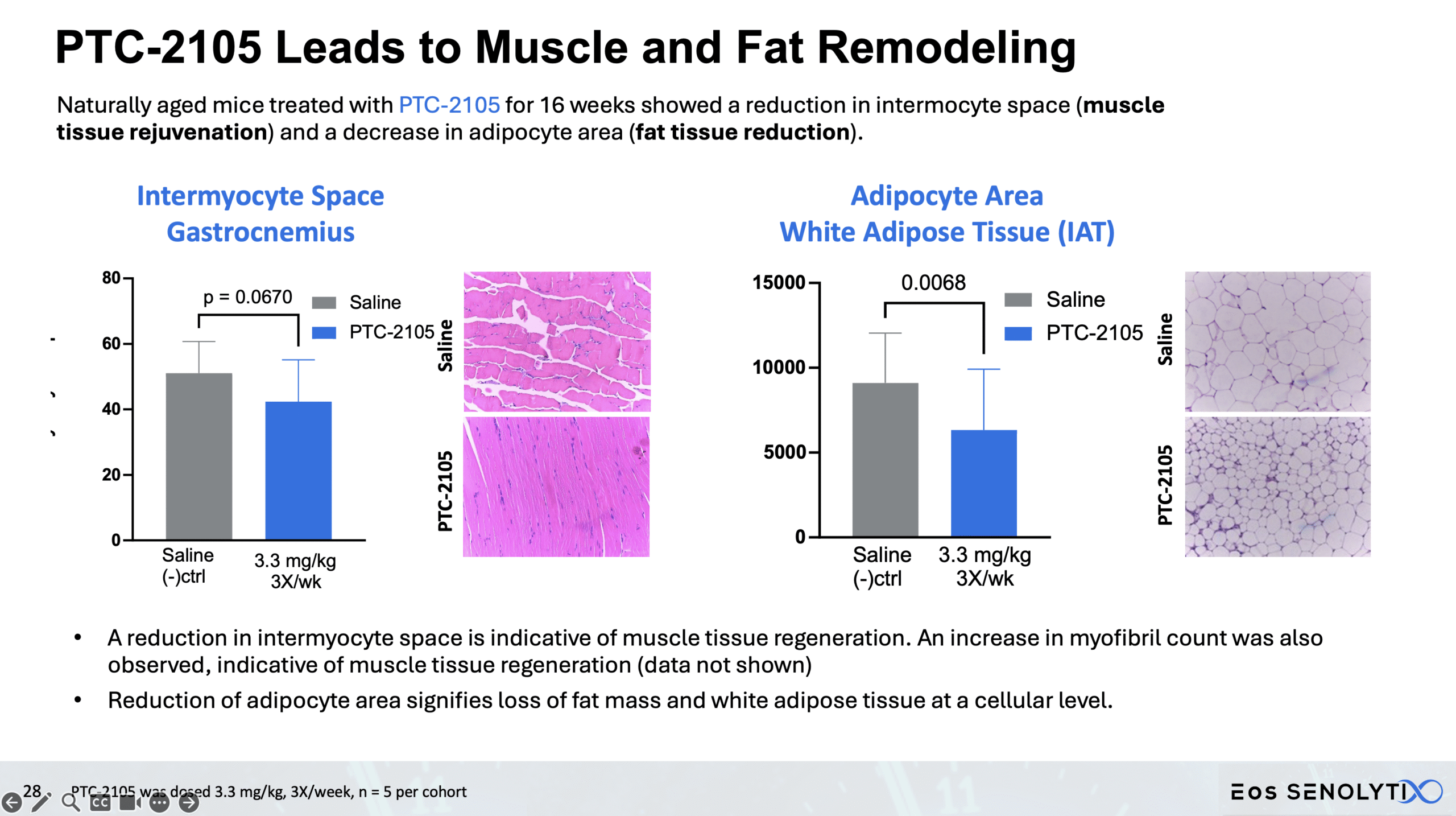
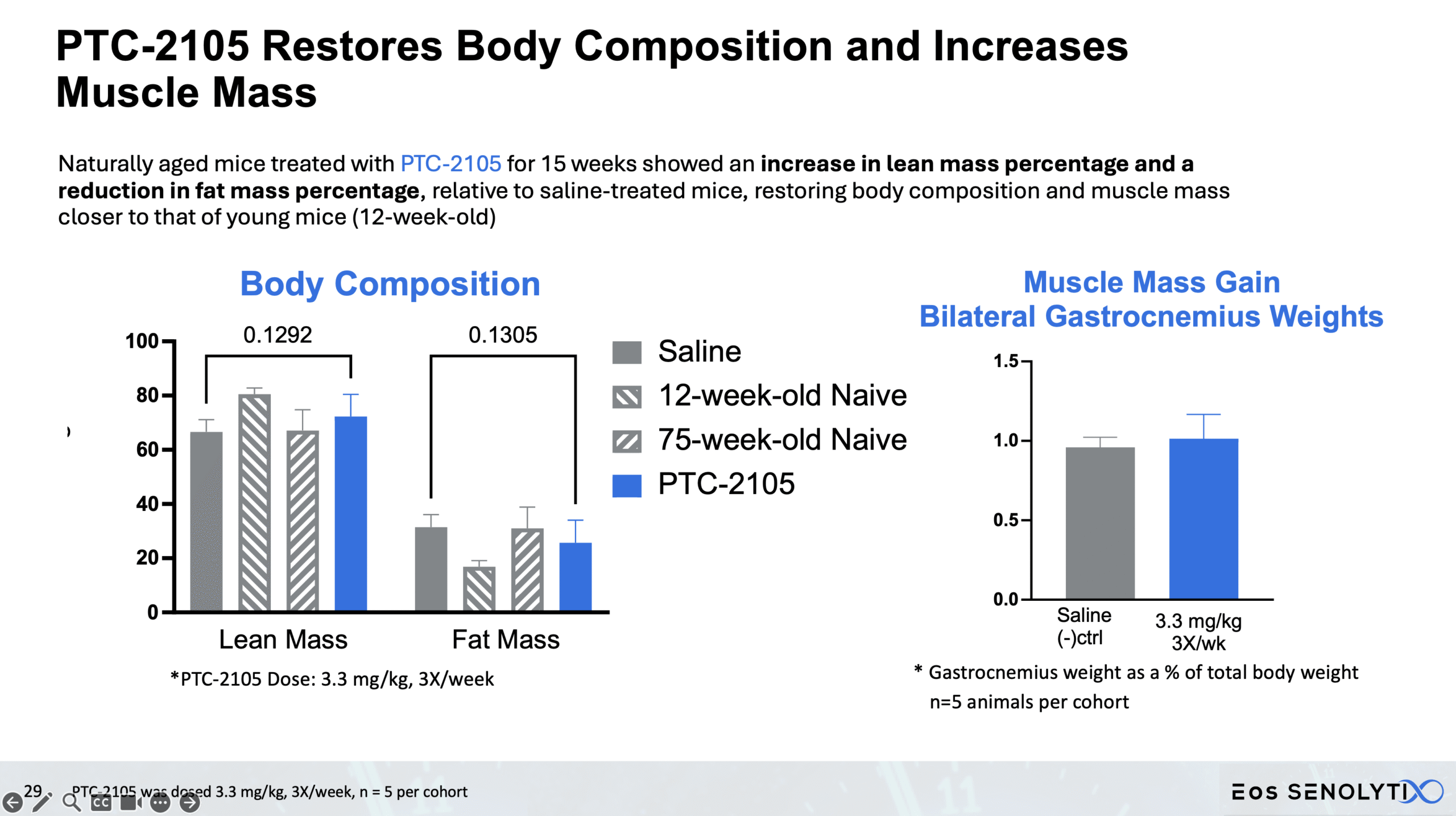
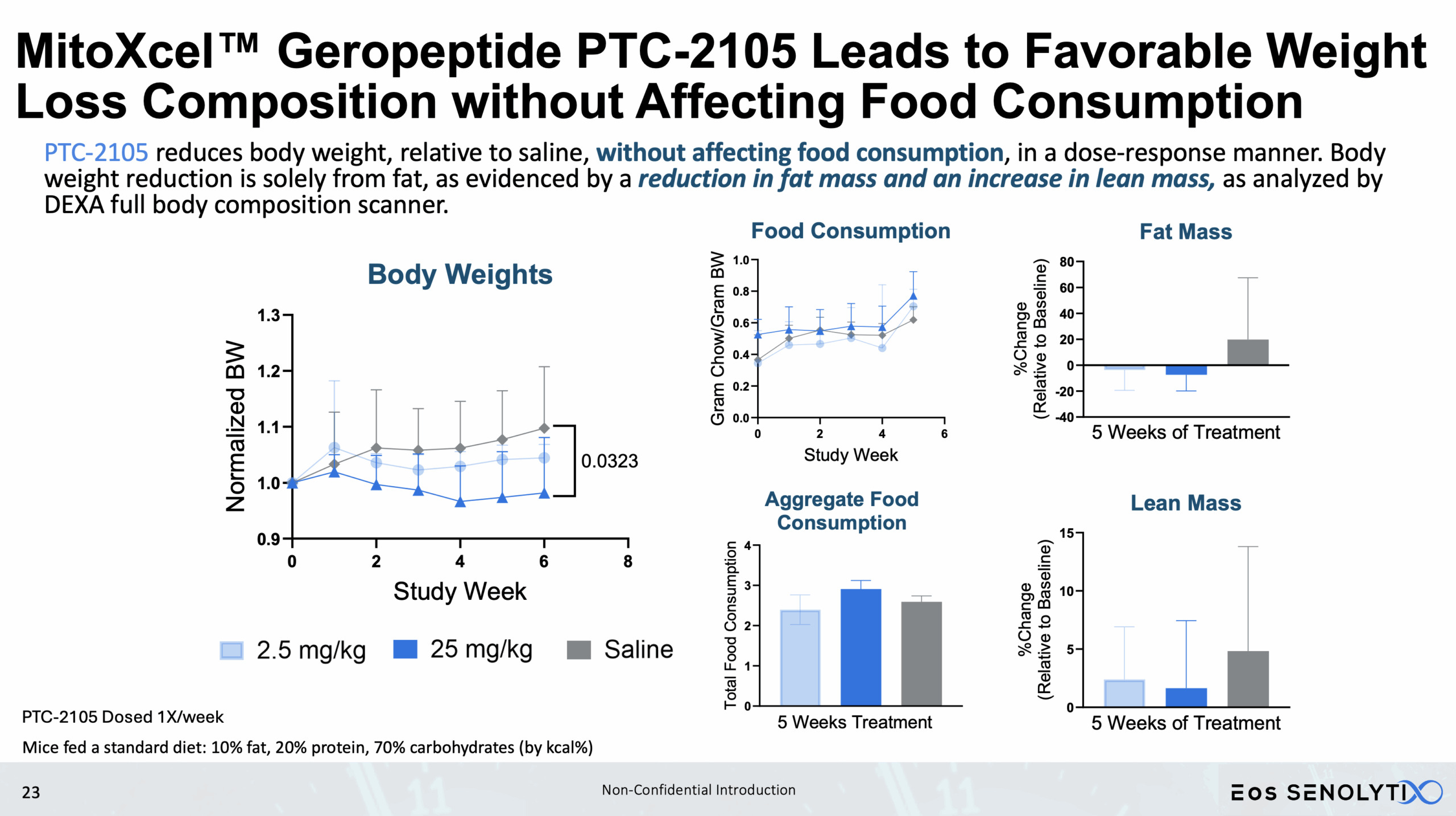
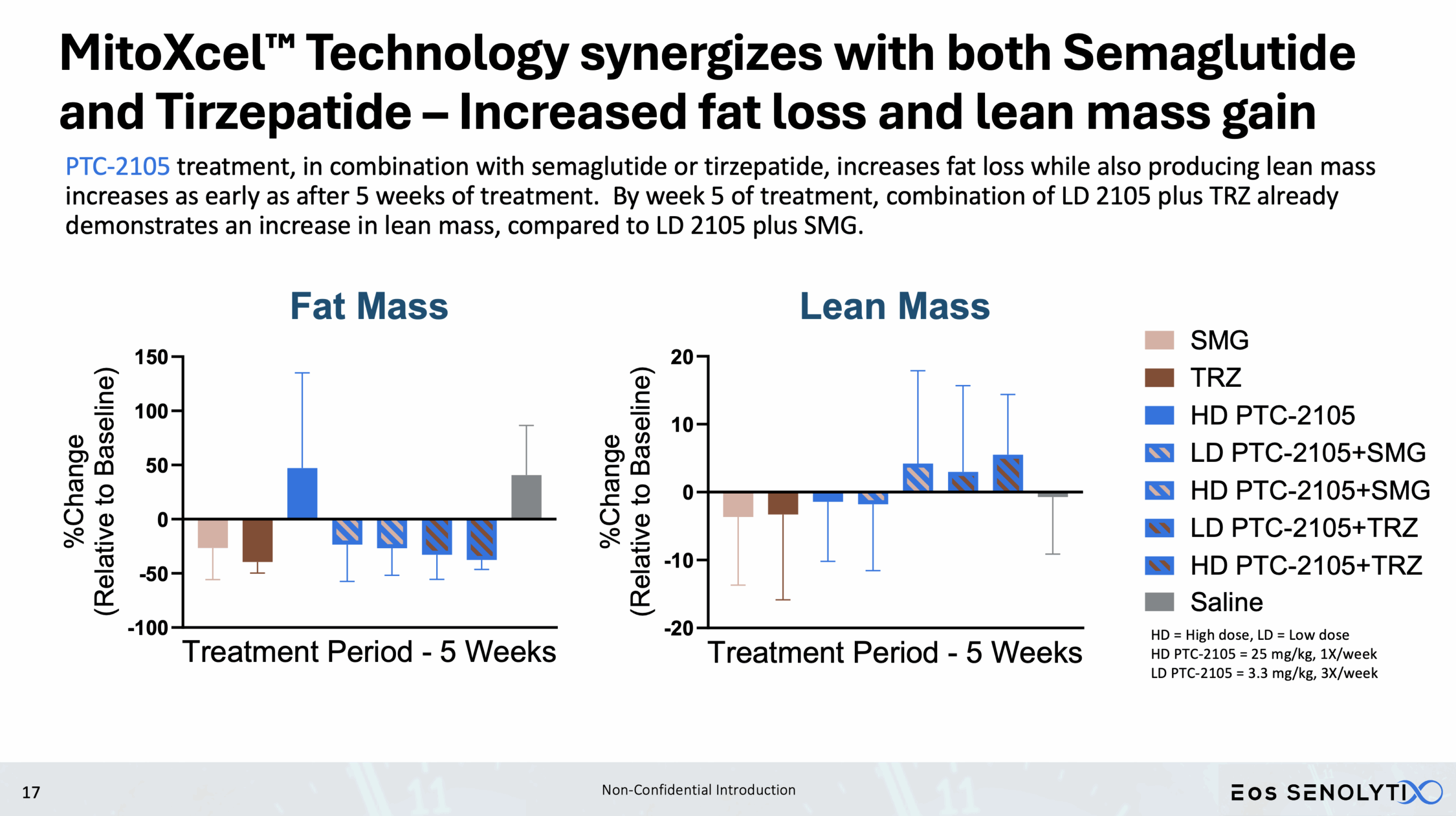
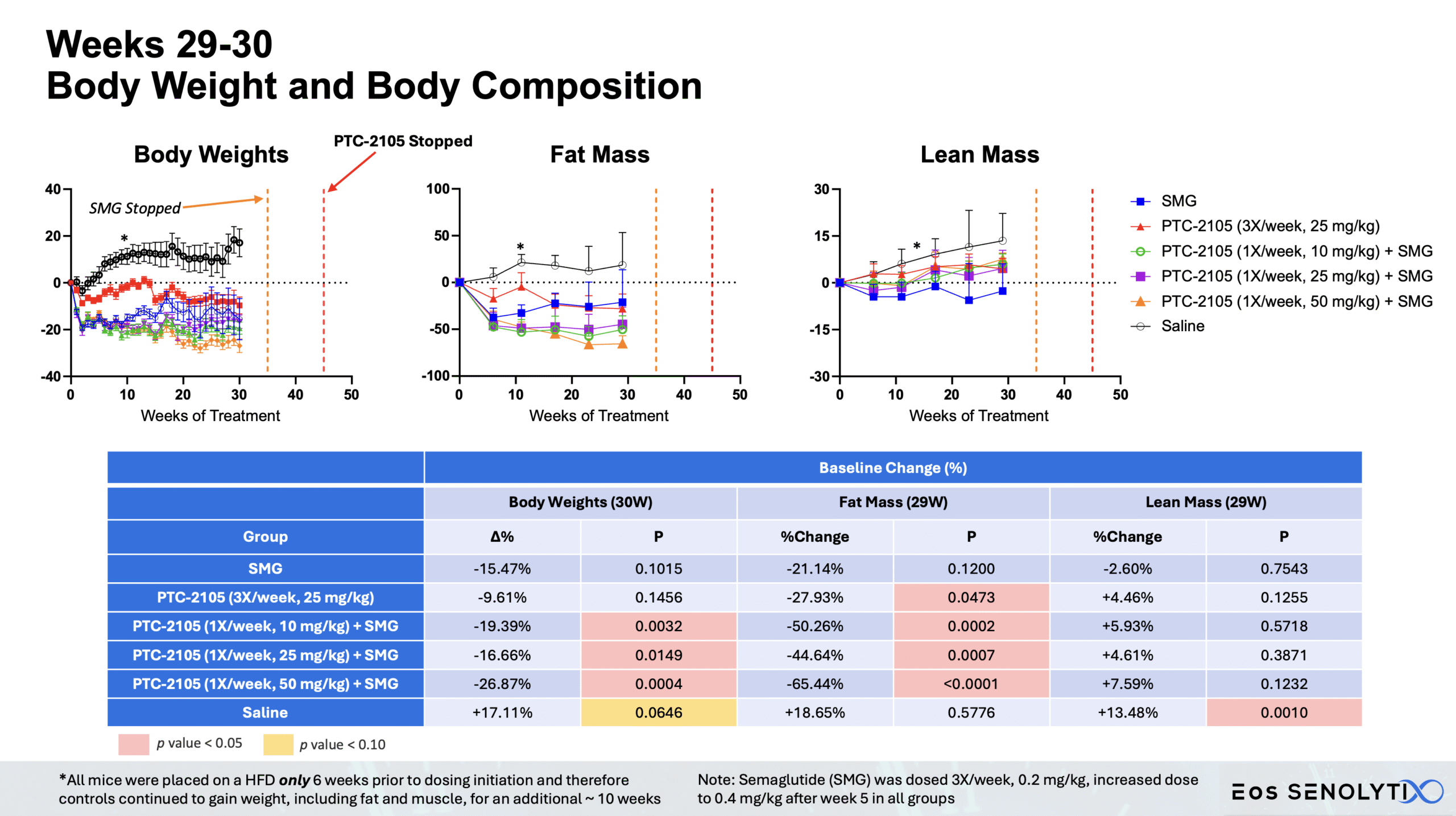
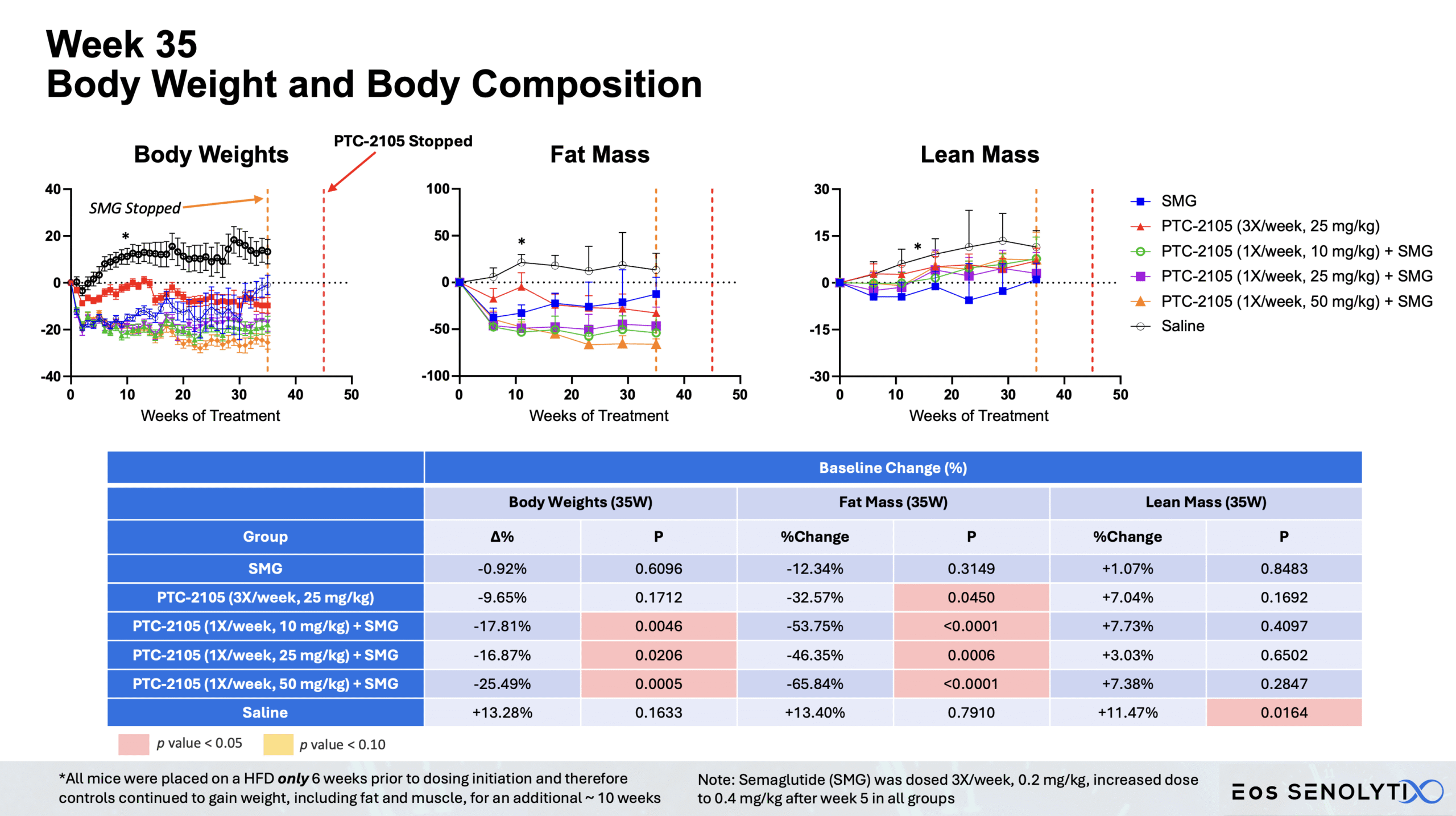
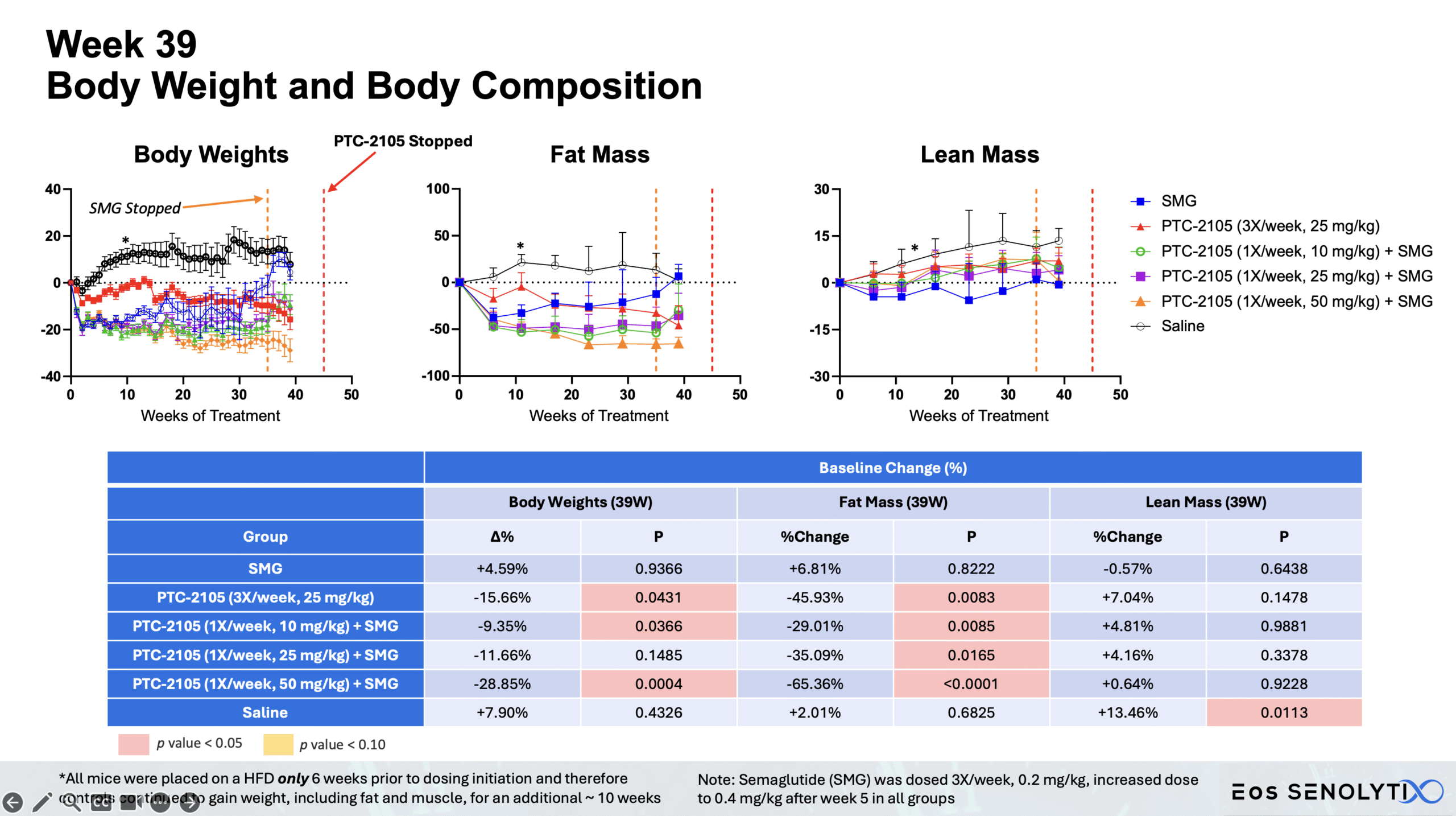
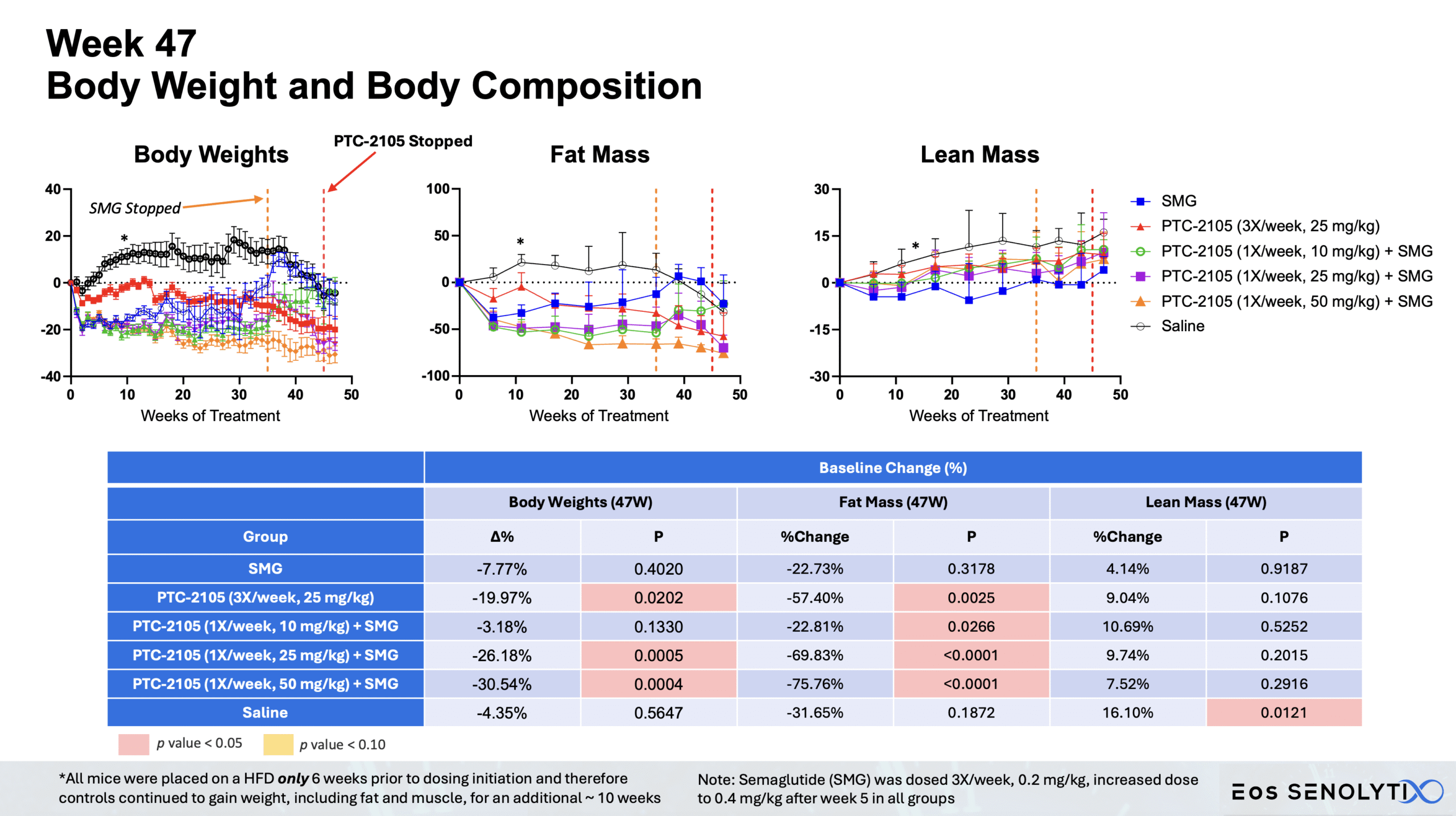
Tissue Remodelling to a Healthier Phenotype in Muscle and Fat: Improved body composition including a reduction in fat but an increase in lean body and bone mass
-
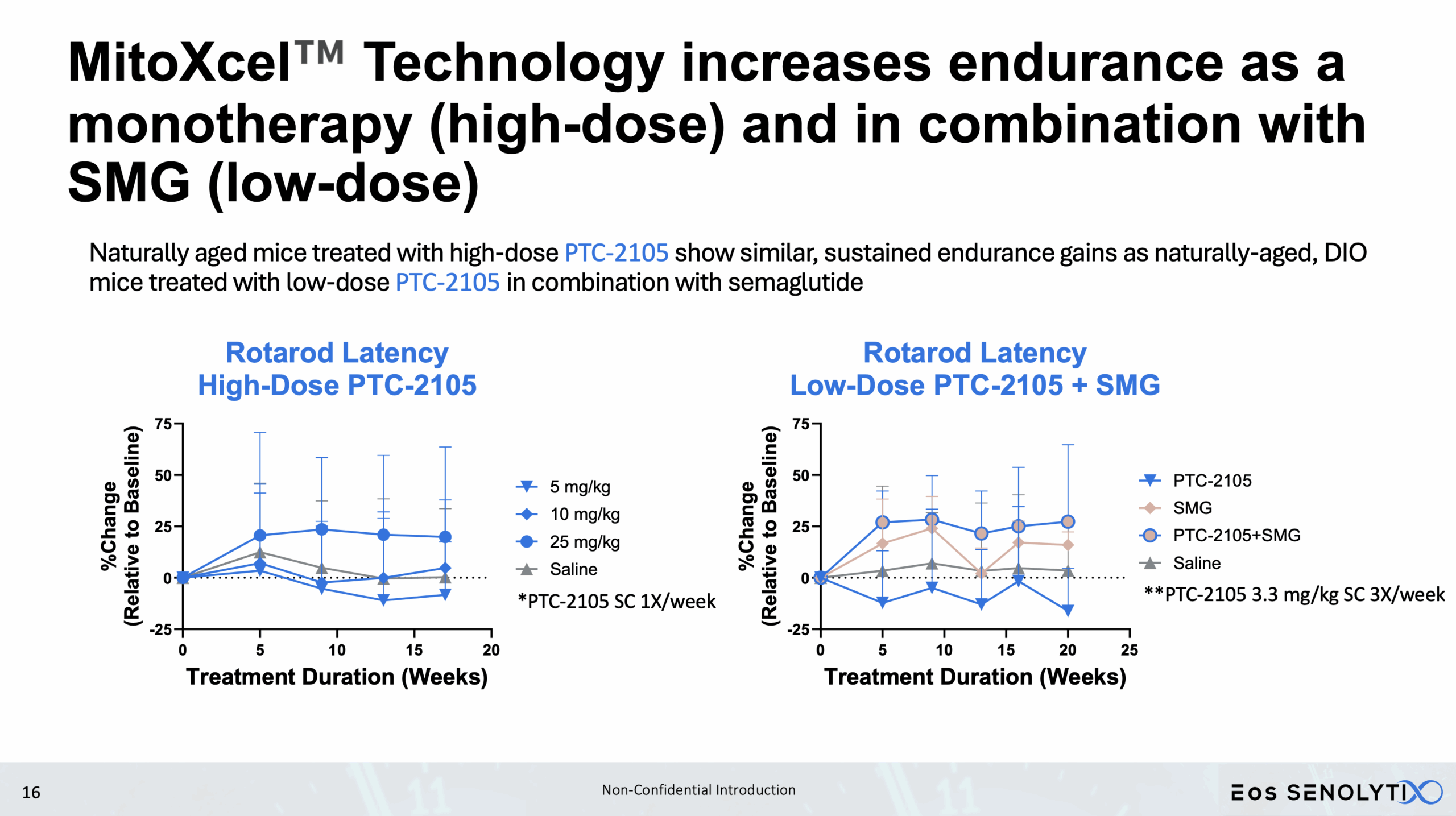
Improved Physical Health: Enhanced metabolism, muscle strength, and exercise endurance.
-
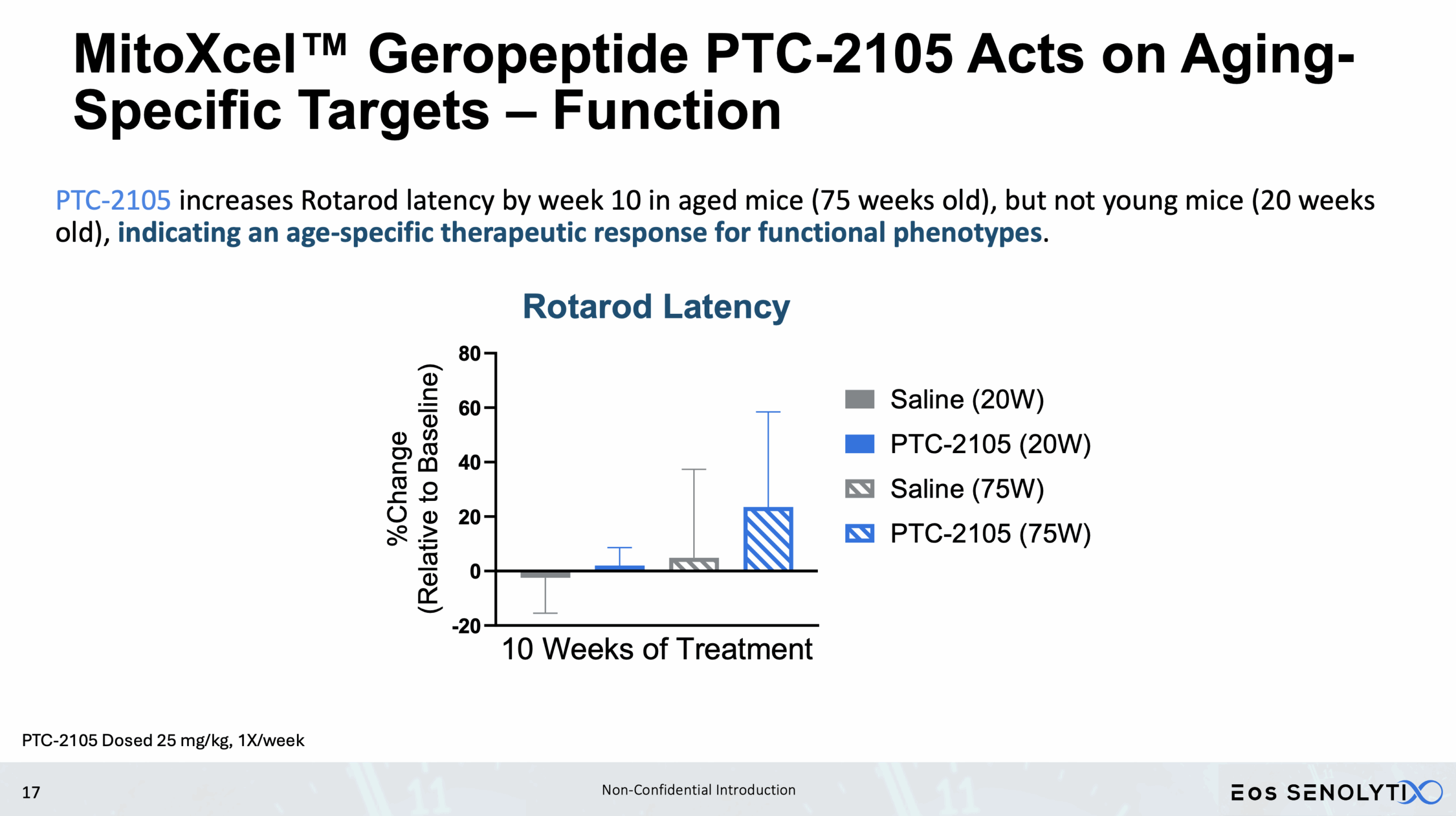
Cognitive Benefits: Improved memory and motor coordination.
-
Dose Response Curve Demonstrated
-
Safety Profile: Without demonstrable evidence of toxicity at >40X the efficacious dose after more than 20 weeks of continuous dosing. Normal liver enzyme levels and no adverse effects observed.
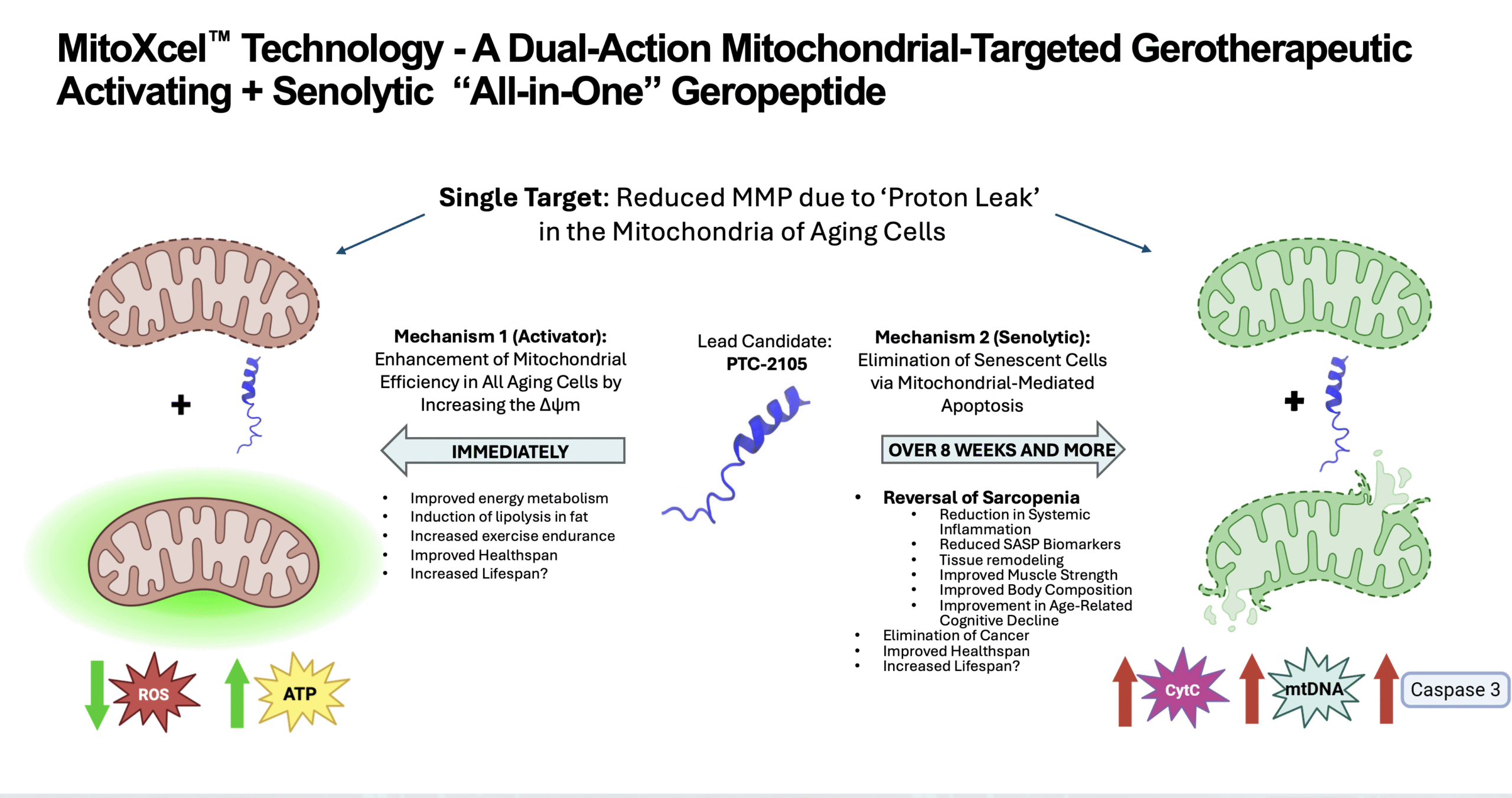
Mechanism of Action: Selectively Target the Inner Mitochondrial Membrane Potential (Δψm) in the Mitochondria of Senescent and Non-Senescent Cells
-
Completely novel, aging-specific mechanism of action
-
Two key mechanisms, both targeting the Inner Mitochondrial Membrane Potential (Δψm)
-
Binds cardiolipin, as well as multiple key proteins involved in the Electron Transport Chain (“ETC”), especially those in Complex III and IV
-
Cardiolipin is crucial for creating and stabilizing the mitochondrial cristae membranes and maintaining IMM architecture, enabling tight packing of respiratory chain complexes.
-
Cardiolipin directly binds to and stabilizes the entire mitochondrial respiratory supercomplex (Complexes I–III–IV megacomplex), functioning to maintain ETC assembly and structural integrity, acting as a molecular glue for oxidative phosphorylation.
-
Under stress, cardiolipin undergoes peroxidation and translocation from the IMM to the outer membrane, triggering Cytochrome C detachment from cardiolipin, the first step of apoptosis.
-
-
This single target leads to two distinct yet equally important mechanisms of action, Mechanism 1 and Mechanism 2
-
Validated in three species: human (in vitro), mouse (in vivo) and C. elegans (in vivo)